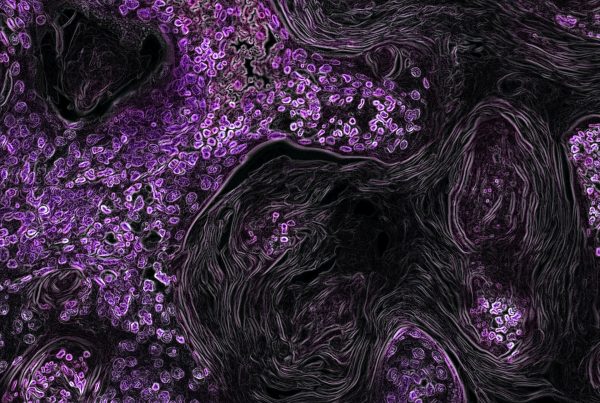
The study examines the effects of increasing cellular NAD+ levels on heart failure. Cardiac dysfunction was induced in SIRT3-deficient mice and compared with wild-type mice treated with nicotinamide riboside chloride. The treatment, started after the onset of cardiac dysfunction, improved mitochondrial function and slowed the progression of heart failure in both mouse types. Benefits included enhanced function of NAD(H) redox-sensitive SDR family proteins and upregulation of Mrpp2, a key component in mitochondrial DNA processing and electron transport chain function. Activation of SDRs also stimulated RXRα/PPARα signaling, enhancing mitochondrial oxidative metabolism. The downregulation of Mrpp2 observed in human failing hearts suggests a common mechanism of mitochondrial biogenesis impairment in both mouse and human heart failure.
But nothing the copy said could convince her
and so it didn’t take long until a few insidious
Copy Writers ambushed her
The results indicate that treatment with nicotinamide riboside chloride enhances mitochondrial function and slows the progression of heart failure in both wild-type and SIRT3-deficient mice. This improvement is linked to the elevated activity of NAD(H) redox-sensitive SDR family proteins and the upregulation of Mrpp2, which boosts mitochondrial DNA processing and electron transport efficiency. Additionally, activation of SDRs enhances RXRα/PPARα signaling, further supporting mitochondrial function. These findings underscore SDR proteins as key regulators of mitochondrial function and viable targets for NAD+-based therapies, effective even after the onset of heart failure symptoms, and independent of the Sirt3-mediated deacetylation previously associated with such treatments.